Multicolored Single-Molecule Imaging of Overcrowding Membrane Proteins
Jan 20, 2015
Newly developed Halo-ligand conjugated fluorescent quantum dots were used for single-molecule imaging of GPCR membrane proteins in living cells. These probes do not inhibit the behavior of target proteins thanks to their small size ( < 6 nm). Individual GPCR proteins within an area of the optical resolution limit (200 nm) can be discriminated by multicolored single-molecule fluorescence imaging with these probes.
Membrane receptors play important roles for cell responses to its surrounding environment. G protein-coupled receptors (GPCR) form the largest family of membrane receptors. Because signaling through GPCR is involved in many biological processes, GPCR malfunction causes various diseases, including diabetes, hypertension, inflammation, cancer, and drug addiction. Currently, nearly half of all drugs available on the market target GPCR proteins.
Biochemical and molecular biological approaches, such as gene cloning and expression, have often been used to study the function of GPCRs in vitro. Recently, single-molecule fluorescence imaging using total internal reflection fluorescence microscopy has been applied to study membrane protein dynamics in living cells. This imaging technique makes it possible to observe the diffusion of membrane proteins at the single-molecule level in real time in living cells to analyze protein function.
There are, however, limitations to this technique. The optical resolution in fluorescence microscopy is restricted by half the wavelength of light, which for visible light means a limit of approximately 200 nm. Thus, molecules within this limit cannot be optically discriminated, but a large number of proteins exist within this range, as their size is normally 5-20 nm. Consequently, only about 10-20% of membrane proteins are observed by conventional single-molecule imaging.
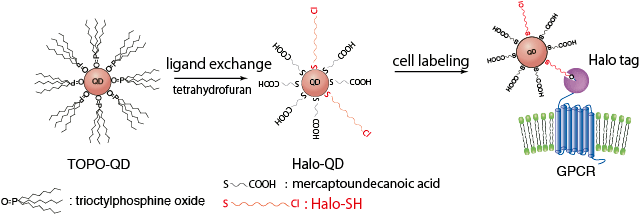
Figure 1. The synthesis of HaloTag-ligand conjugated QDs and labeling of HaloTag protein-fused GPCR on the cell surface.
In a new paper published by the Laboratory for Nano-Bio Probes and the Laboratory for Cell Signaling Dynamics at QBiC, Takashi Jin and his team has reported a multicolored single-molecule imaging technique to investigate the dynamics of GPCR proteins in living cells with a resolution that surpasses the optical limit [1]. Key to this success was the use of quantum dots (QDs) as the fluorescent probe. QDs have superior optical properties, such as high fluorescence brightness (ten times stronger than that of organic dyes) and higher resistance to photo-bleaching than traditional dyes. The authors synthesized compact QDs no more than 6 nm wide that emit bright fluorescence from the visible to near-infrared region. Furthermore, the particle size can be tailored to produce emissions at different wavelengths.
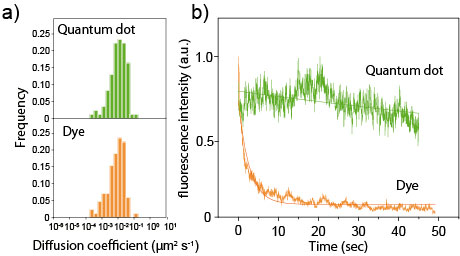
Figure 2. (a) Diffusion coefficients of cAR1 receptors, as determined by single-molecule tracking using QDs or organic dyes (tetramethyl rhodamine). (b) Fluorescence photobleaching of Halo-QDs and organic dyes (tetramethyl rhodamine).
QD-based probes were prepared by conjugating HaloTag ligands to their surface. The ligands were designed to covalently bind to GPCR-HaloTag fusion proteins (Fig.1). With this QD probe, the team labeled cAR1, a GPCR for extracellular cyclic AMP in Dictyostelium discoideum cells. Due to their small size, the QD probes did not inhibit the behavior of the target protein. Furthermore, because the QD probes were highly fluorescent and resistant to photo-bleaching, long-term observation of single-molecules was made (Fig.2). By using three types of QD probes, those that emitted green, red, or near-infrared fluorescence, the team achieved multicolored single-molecule imaging of cAR1 membrane proteins and detected overcrowding proteins (Fig.3).
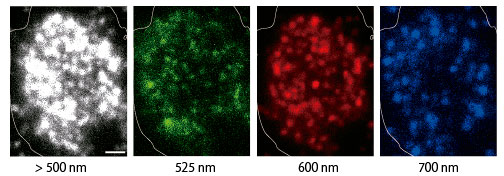
Figure 3. One-colored (> 500 nm) and multicolored (green, red, and blue; with emission filters) single-molecule fluorescence images of QD-labeled HaloTag fusion cAR1 receptors in a Dictyostelium cell. Scale bar: 1 μm.
“We could detect three times more cAR1 molecules by using multicolored single-molecule imaging than by one-colored single-molecule imaging ” explains Akihito Komatsuzaki, the lead author of the paper. “We could demonstrate that multicolored single-molecule imaging is a powerful tool to observe overcrowding proteins on cell membranes.”
In addition Komatsuzaki emphasized the user friendly and versatile features of the probe. “This technique can be readily applied to any HaloTag-fusion protein without the need for super resolution microscopy”
He expects that the technique will be applied to the investigation of various membrane receptor mediated cellular processes including immune-inflammatory responses and neurotransmission.
- A. Komatsuzaki, T. Ohyanagi, Y. Tsukasaki, Y. Miyanaga, M. Ueda and T Jin, “Compact Halo-Ligand Conjugated Quantum Dots for Multicolored Single-Molecule Imaging of Overcrowding GPCR Proteins on Cell Membranes" Small, 2014. DOI:10.1002/smll.201402508

