
TOP > News & Articles > Research Highlight > A look inside the see-through mouse
A look inside the see-through mouse

A new method developed by QBiC researchers makes it possible to turn an entire mouse transparent. Combined with staining techniques and computer-assisted image acquisition, this approach lets scientists study cellular networks in animal brains and reconstruct the anatomy of internal structures of various organs in 3D.
Inside a small vial, the trace of what once was a baby mouse floats like a pale ghost in a clear solution. The only visible remnants of its body are the bones, the iris pigments, and whitish sac in its abdomen. "That is the milk the pup took into its stomach. The reagent somehow did not render the milk proteins transparent," explains Kazuki Tainaka, who brought this science fiction-like technique to fruition. The see-through mouse is amazing to behold, but even more wonderful is the role serendipity played in making this a reality.
More than 20 medical students are working in the new home of the QBiC Laboratory for Synthetic Biology in the University of Tokyo main campus. The lab, headed by Hiroki R. Ueda, a pioneer in systems biology, previously focused on the identification and analysis of system components and their interactions. Recently appointed professor in the Faculty of Medicine, Ueda continues to promote systems biology research at the organismal level, now aiming to combine basic science and medical technology. The team has invested heavily in technologies for modifying genes, by high-throughput generation of genetically modified mice and analyzing tissue- or organism-level systems. Tainaka, a chemist by training and QBiC research scientist for much of the project, played a central role in the effort to develop the transparent mouse; he now works as a lecturer at the University of Tokyo. In 2014, he and Etsuo Susaki, medical scientist in the lab, published a report in Cell describing a cocktail of reagents capable of making mouse and marmoset brain tissue transparent [1].
"We were interested in mammalian circadian rhythms, which appear in different levels from the molecular and cellular to the whole-body level", explains Tainaka. "Previously we studied molecular networks that display such rhythms in cells. But at the organism level, in the sleeping cycle for example, these rhythms arise from the cellular networks in the brain. A few methods had been developed for clearing individual brain sections, but we felt there was still a need for an improved method for whole-brain imaging at single-cell resolution."
The group suspects that the transparency is achieved by minimizing light scattering at the surface of brain. Because lipids are major source of light scattering, the removal of lipids and/or adjustment of the refractive indices of tissues and the surrounding solution to the same levels are potential approaches for making brain optically transparent. They tested various chemical cocktails to determine which combination is most effective in clearing tissue. The group painstakingly designed their screen by mixing homogenized fixed brain with chemical cocktails in small test tubes and measuring refractive indices. They found that aminoalcohol derivatives were highly effective for making the tissue clear. The reagents’ pH was also optimized so as not to quench fluorescent proteins, which were used for labeling cells. Combined with computer-assisted image acquisition, the method, which they named CUBIC for Clear, Unobstructed Brain Imaging Cocktails and Computational Analysis, enables whole-brain imaging at single-cell resolution. These results were published in Cell in April 2014 [1].
Just a month before they published that article, a researcher in a neighboring lab borrowed the reagent and hinted that the reagent could be used for clearing the other organs as well. Tainaka tested the possibility, and soon found that the CUBIC technique indeed decolorized organs other than the brain. "We never thought that other organs could be so easily cleared, since unlike the brain they include chromophores, such as hemoglobin in blood. But we found that the same reagent somehow makes any tissue other than bones and pigments transparent. This was totally unexpected, and a pleasant surprise."
After discovering this more wide-ranging effect, he began working to tease out the mechanism of decolorization. It turned out that the cocktail can efficiently elute heme chromophore from hemoglobin, which had previously been assumed to be tightly associated. To minimize quenching of fluorescent proteins, the CUBIC pH had been tweaked to a slightly alkaline level, which also happened to be the optimal pH for eluting heme from globin. Over an 8-month period, the group managed to assemble their new data and submit another manuscript describing the general applicability of CUBIC to Cell [2].
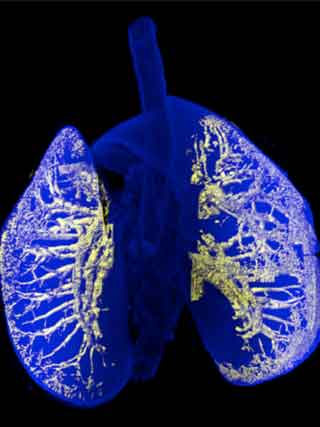
3D reconstruction of mouse lung bronchi by CUBIC method.
The initial aim of this study was to visualize networks in brain. But now the group is able to clear an entire mouse body, making it possible to visualize the internal structures of various tissues by immunostaining or expressing fluorescent proteins. The Ueda group also developed a computer program that analyzes staining and extract 3D internal structures, such as bronchi in lung and Langerhans islets in pancreas. Traditionally, 3D anatomical images of internal structures have been reconstituted from a number of serial sections, which is possible only by highly experienced specialists. "Now anyone can make accurate 3D anatomical reconstruction without needing to dissect tissues", explains Tainaka. "This is a very nice advantage, especially in a medical school"
The creation of a transparent mouse has been featured in newspapers and online publications worldwide. "Lots of reporters, even TV stations came to the lab, but I am not the kind of person who likes to be in the spotlight. Now I just want to go back to my experiments as soon as possible," says Tainaka.
- E. A. Susaki, K. Tainaka, D. Perrin, F. Kishino, T. Tawara, T. M. Watanabe, C. Yokoyama, H. Onoe, M. Eguchi, S. Yamaguchi, T. Abe, H. Kiyonari, Y. Shimizu, A. Miyawaki, H. Yokota and H. R. Ueda. “Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis.” Cell 157(3): 726-39(2014)
- K. Tainaka, S. I. Kubota, T. Q. Suyama, E. A. Susaki, D. Perrin, M. Ukai-Tadenuma, H. Ukai, H. R. Ueda. “Whole-body imaging with single-cell resolution by tissue decolorization.” Cell 159(4): 911-24(2014)

