How chemotactic cells extend the response range
Apr 18, 2016 Tweet
Cells can move along a chemical concentration gradient in a phenomenon called "chemotaxis". This phenomenon plays important roles in embryogenesis, immune response, neuronal wiring, as well as in wound healing. A soil microorganism (Dictyostelium discoideum) known as a slime mold has been used in the research of chemotaxis. The “cellular” slime molds usually feed on bacteria, but when starved of nutrients they exhibit chemotaxis and aggregate. The cells move in attraction to a secreted cyclic adenosine monophosphate (cAMP) produced by themselves. When the chemoattractant cAMP binds to a G protein-coupled receptor (GPCR) on the cell membrane, trimeric G proteins are activated to transmit signals downstream. Through the control of actin cytoskeleton, the cell moves. Furthermore, chemotactic cells generally recognize a very small density difference as small as a few percent of chemoattractant over a concentration range as much as 100,000 fold. This remarkably flexible ability of cells has attracted attention from researchers for long time.
Yoichiro Kamimura, senior scientist, Yukihiro Miyanaga, visiting scientist and Masahiro Ueda, Group Director of the Laboratory for Cell Signaling Dynamics discovered a new protein factor that regulates the response range of chemotaxis in D. discoideum and named the factor Gip1 (trimeric G protein interacting protein 1). The discovery is highlighted in their latest paper published in Proc. Natl. Acad. Sci. U.S.A.[1]
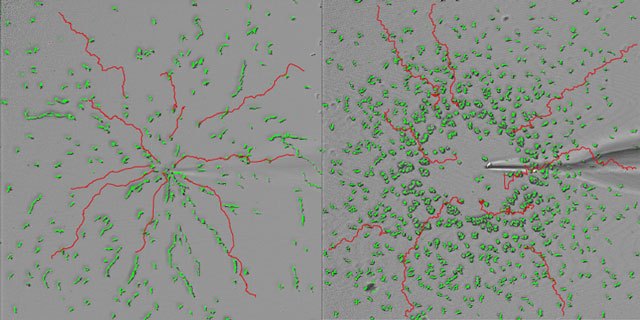
When the cellular slime molds are placed in a concentration gradient of cAMP emitted from the tip of the micropipette the molds move linearly toward the high concentration center (Picture left). On the other hand, the strains that are genetically deficient of Gip1 cannot maintain directed movement at a high concentration of cAMP (right). This result indicates that Gip1 is a factor needed to maintain the response to the concentration gradient of the chemoattractant at high concentrations.
Although G proteins function on the cell membrane, some fraction localizes in the cytoplasm through the binding to Gip1. Upon stimulation with chemoattractant, G proteins dissociate from Gip1 and move to the cell membrane. Even at high concentrations of chemoattractant, appropriate amounts of G protein are thought to be supplied to the cell membrane. The supply comes from the reservoir of G proteins bound to Gip1 in the cytoplasm. In this way a spatial deviation of G proteins on the cell membrane is formed in accordance with the concentration gradient of the chemoattractant. Thus even high concentrations of chemoattractant cells properly sense the concentration gradient and are able to conduct directed movement. According to Kamimura, “These findings shed light on a new regulatory mechanism of G proteins by intracellular distribution.”
Looking to the future he suggests, “This mechanism could be harnessed for broad dynamic range regulation that is a frequently observed feature of sensory cells as well as a seed growing into a new molecular tool manipulating G proteins activity.”
- Kamimura,Y.,Miyanaga,Y., and Ueda, M. “Heterotrimeric G protein shuttling via Gip1 extends the dynamic range of eukaryotic chemotaxis.” Proc. Natl. Acad. Sci. U.S.A. doi:10.1073/pnas.1516767113

