TOP > News > Research Highlight > Nano-probes for intracellular viscosity

![]()
Aug 16, 2012
Nano-probes for intracellular viscosity
Quantum dots (QD) are tiny semi conductors that through modification can now be used as probes for the measurement of biological phenomena. Their appeal in biological studies is that they offer a higher fluorescent signal and slower photobleaching time than traditional fluorescent probes, which allows for an increased observation time of the phenomenon of interest. Furthermore, the metal core and size of a QD can be modified accordingly to maximize the fluorescence intensity, as too can the wavelength, making QD inordinately applicable to a wide range of biological studies.
When measuring biological phenomena, QD are commonly combined with techniques like single particle tracking, which requires the QD be directly attached to the protein of interest. Unfortunately, because of this attachment, the intracellular environment cannot be directly measured, only the molecule’s response to it. Takashi Jin and his team at the Laboratory for Nano-Bio Probes, however, have been able to formulate QD that can measure cellular properties even when unbound to a molecule, a development illustrated on the cover of Analytical Methods (Royal Society of Chemistry)1. By coating their QD with bovine serum albumin (BSA) and injecting them into HeLa cells, the group could measure the free diffusion of their BSA-QD by fluorescence correlation spectroscopy (FCS) and therefore evaluate the viscosity of the intracellular environment (Fig. 1). This ability very much depends on the spherical shape of the BSA-QD, which allows the team to apply the Stokes-Einstein equation to calculate the corresponding diffusion constants.
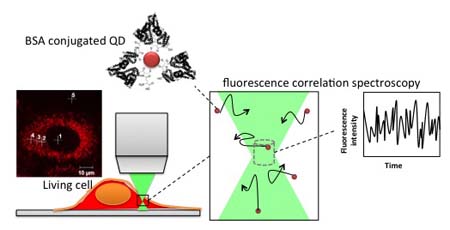
Figure 1. BSA-QD was introduced into a living cell and cytoplasmic viscosity was measured by fluorescence correlation spectroscopy.
The group found that unlike other coats, BSA was crucial for the QD to disperse throughout the intracellular environment. Autocorrelation curves of the BSA-QD behavior showed a fast and a slow constant, which were attributed to QD free in the cytoplasm and QD associated with cytoplasmic elements like the cytoskeleton, respectively. The BSA-QD also showed diffusion properties and viscosities that resembled a previous report using rhodamine-green labeled DNA. FCS results of BSA-QD indicated intracellular viscosities that were 4.7 times greater than that of water, which is in agreement with the rhodamine-green labeled DNA study that found the ratio to be 4.8.
Yet if rhodamine-green can already be used to measure viscosity, what is the innovation in the design of these QD? The two probes showed similar sizes: 9.1 nm for the BSA-QD and 10.9 nm for the rhodamine-green labeled DNA. However, first author Yuko Nakane explains that while the two probes have similar sizes, the shape of the BSA-QD makes it a more reliable tool for measuring intracellular properties like viscosity. “The important difference is that rhodamine-green is not spherical whereas our QD are”. The reason this distinction is important is because although viscosity depends on size, the shape of the particle can also have an effect, making it difficult to compare viscosities if both the size and shape of two particles vary. On the other hand, the Jin group can control for shape. “Manufacturing and testing different sized spherical QD means we can develop a table that relates viscosity and size and therefore collect more reliable data about the cell”.
Designing QD of different sizes and applying the same analysis then should allow scientists to better model viscosity effects when experimental measurements cannot be made.
- Yuko Nakane, Akira Sasaki, Masataka Kinjo and Takashi Jin. Anal. Methods, 2012, 4, 1903-1905

