TOP > News > Research Highlight > Right Appetite for the Growth
![]()
Right Appetite for Growth
Living creatures have the remarkable ability to retain their function even when the surrounding environment changes. For example, our body sweats when we are hot, our pupils dilate when we are in the dark, and our metabolism slows when we sleep. Analogously, cells too must respond to environmental changes. These cellular responses often arise by changing the dynamics of a cell’s chemical reactions.
One way cells do this is by continuously absorbing nutrients that are then processed into energy for growth and survival. Chikara Furusawa of the Laboratory for Multiscale Biosystem Dynamics, with his colleague Kunihiko Kaneko, shows using a mathematical model that the dynamics of these processes self-organize into a critical state so that that the cell can absorb only the amount of nutrient needed to maintain the optimal growth rate (http://prl.aps.org/abstract/PRL/v108/i20/e208103). The authors found that nutrient absorption by the cell reaches a steady state at a certain extracellular concentration of the nutrient, which suggests the cell reduces the concentration of the transporter responsible for recruiting the nutrient. In other words, so long the extracellular concentration of the nutrient exceeds some critical value, a self-regulation mechanism is activated and modifies the cellular dynamics so that the cell intakes only the amount of nutrient needed for optimal growth.
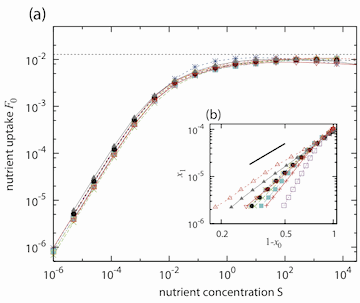
Figure 1. There exists a critical state that nutrient uptake cannot exceed for the cell to sustain optimal growth.
To better understand the mechanism regulating nutrient uptake, Furusawa and Kaneko considered the downstream chemicals that are derived. At low nutrient levels, the reactions occur faster than the nutrient uptake, meaning that all chemicals have approximately identical concentrations. However, as the nutrient uptake increases, especially to values near the critical value, they found that the chemical distributions begin to approximate the power law, a result that agreed with experimental data. Their results argue that the mechanism used by the cell to self-organize is independent of the method in which the nutrient enters the cell.
The authors confirmed this finding by applying a mean-field calculation, where they looked at the chemical reactions that occur at each tier of the pathway, beginning from the nutrient to the final chemical produced. This method also showed that the critical state is independent of sufficiently large nutrient concentrations and therefore confirms that the cell self-organizes to sustain optical growth.
Moreover, the two features of a cell’s response to environmental changes are revealed. It is well known that chemotactic cells respond not to absolute concentrations of a signal to regulate their direction of movement, but rather to changes in the concentration. Such responses are termed fold-change detection, since the cell’s response is proportional to the changes in the signal. Furusawa and Kaneko found similarly that the response to environmental changes to recover the critical state depends only on the change in the extracellular concentration of the nutrient and that downstream chemicals derived from the nutrient are accordingly modified with changes in this value.
The findings from their work suggest that self-organization in response to nutrient fluctuations is a paradigm for how cellular systems function with a high degree of flexibility and robustness, as the power law properties described here are seen in many cellular and even genetic processes.
“Although the mechanism of adaptation we proposed is quite simple, it can be universal in replicating cellular systems,” Furusawa says. “This mechanism might exist behind sophisticated regulatory programs to maintain robust adaptation dynamics.”
- Furusawa, C. and Kaneko, K.(2012) Adaptation to Optimal Cell Growth through Self-Organized Criticality: Phys. Rev. Lett. 108, 208103

