We are interested in cellular functions such as intracellular information processing and cell motility. In particular, we focus on questions such as `how do these cellular properties spontaneously arise from biomolecules and reaction networks composed of biomolecules?’, and `what are the mechanisms that enables the information processing system to be robust to intrinsic noise, and sometimes utilize the noise to express its functions while also receiving severe stochastic fluctuations from the external environment?’.
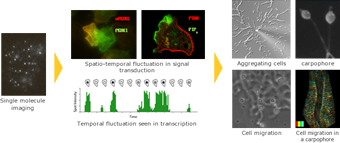
As a typical example of such a stochastic calculation process, we focus on concentration gradient sensing and taxis of Dictyostelium discoideum. Our research group utilizes cutting-edge measurement techniques such as single-molecule imaging, theoretical approaches including mathematical modeling with the aid of high-performance computers, and in vitro reconstitution of minimal molecular reaction systems. We aim to understand the design principles underlying the remarkable information processing capability of cells.
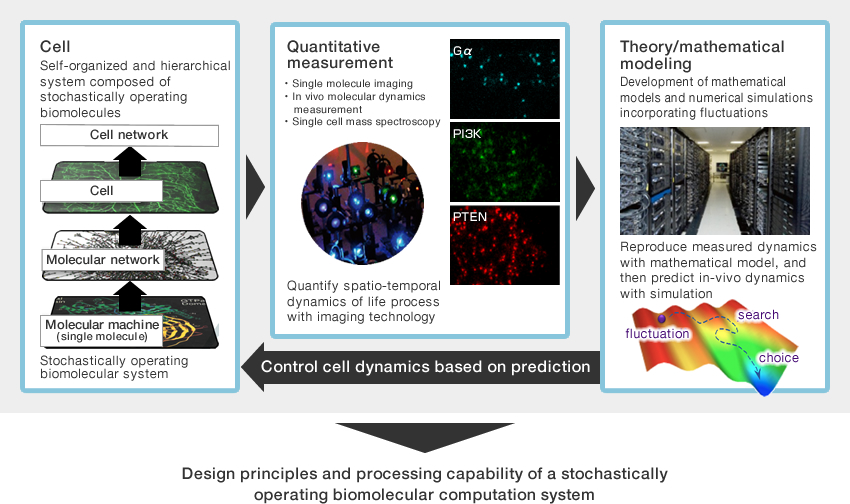
The cell —a self-organized hierarchical system
Biomolecules undergo thermally-driven stochastic fluctuations. The stochastic characteristics of biomolecules generate noise in the course of intracellular signal transduction, and cells exhibit fluctuating responses and spontaneous random walk movements even when in a homogeneous environment. Despite this, cells can adapt to a nonconstant environment, and band together to form or sustain an organism. We call this characteristic ‘organized randomness’, a feature seen at many levels in a living system.
We treat the cell as a self-organized hierarchical system. We study the mechanisms that underlie the dynamics measured at molecular to organismal scales, and that lead to the emergence and regulation of behaviour across these levels of spatiotemporal organisation.
We treat the cell as a self-organized hierarchical system. We study the mechanisms that underlie the dynamics measured at molecular to organismal scales, and that lead to the emergence and regulation of behaviour across these levels of spatiotemporal organisation.
Quantitative microscopy —to measure the dynamics of cells.
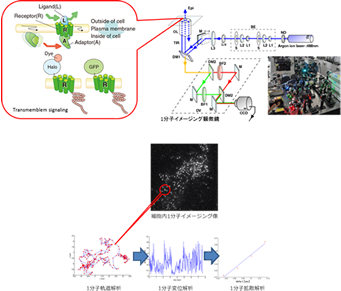
We are developing several imaging and analytical techniques, including In cell Single molecule Imaging (ISI), and combing these techniques with others that measure the bulk behaviour of molecular collectives (e.g. confocal microscopy). We quantify physiological parameters such as molecular diffusion constants, membrane dissociation kinetics, and intramolecular domain motion. We then have direct information about the fluctuating molecular dynamics that form the substrate of cellular functions.
We can realize ‘In cell single molecule imaging’ using objective lens type TIRFM (total internal reflection fluorescence microscopy). Evanescent field light illumination injures cells less than transmitted light illumination.
Mathematical modeling —Bridging the gap between cellular dynamics and understanding
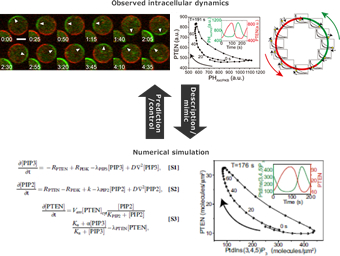
Observed cellular dynamics are subjected to mathematical modeling. A model that reproduces the cellular dynamics would give a quantitative description of the dynamics on the basis of underlying kinetics such as biochemical reactions and diffusion. We reveal how cellular functions such as information processing and motility emerge from its components.We are also trying to see whether fluctuations emerging at each level of organisation serve to enhance or hinder cellular function at other levels by analysing the mathematical models.
Through these processes, we strive to understand the cell as an innate fluctuating system with simple and unified rules.