Research
- Previous project
- Current project
Previous project
Molecular mechanism of motor protein, myosin
I have been focusing on the unique properties of nature's nano-machine using motor protein (myosin) as a model system.
Motor protein is a 10-nm size molecule and achieves force generation or directional motion under Brownian noise which is inevitable in the nano world. Input energy to drive motor protein is supplied from ATP (Adenosine Triphosphate) molecules. Although man-made system such as semiconductor devices uses huge amount of input energy (108 kBT/bit) to completely shut down the Brownian noise, motor protein cannot shut down the noise due to the low input energy of ATP (~ 20kBT). Therefore, these nanomachine must deal with the noise in a different manner compared with man-made system and achieve physiological function.
There would exists various logic to get along with thermal noise and stochastic behaviors at each biological hierarchy, but I have been approaching the issue at the molecular level by refining the single molecule measurement techniques (optical tweezers, single molecule imaging)
My notable results are as follows.
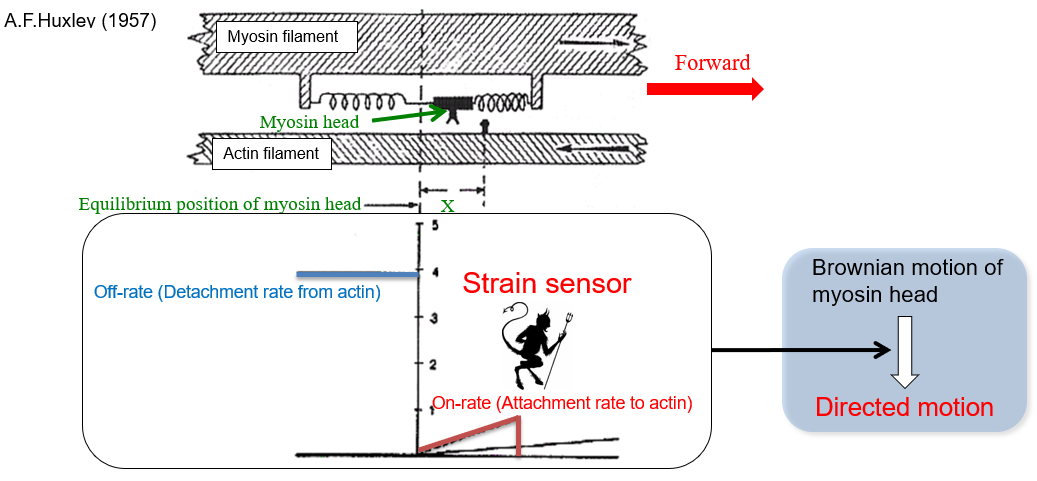
1. Strain sensor mechanism of myosin as a rectifier of random Brownian motion
Detail is linked here but briefly, I focused on the essential point of force generation mechanism, that is, How do myosin determine the directionality? or What's the symmetry breaking mechanism? We found that strain-sensing mechanism in the motor domain rectified the random Brownian motion, resulting in a directional motion along actin tracks. The phenomenon is apparently similar with Maxwell's demon in that work is produced from random motion. Therefore, strain sensor acts as a demon. Although the strain-sensing is coupled with the chemical cycle of ATP hydrolysis (Pi release), which means the mechanism doesn't violate thermodynamic low, we clarified the biological molecular motors called myosin-V and VI are Brownian molecular machine. The input energy of ATP is consumed by keeping the motor in the forward.
Reference
- “Brownian search-and-catch mechanism for myosin-VI steps.”
M. Iwaki, A. H. Iwane, T. Shimokawa, R. Cookes and T. Yanagida
Nature Chemical Biology 5:403-405 (2009)
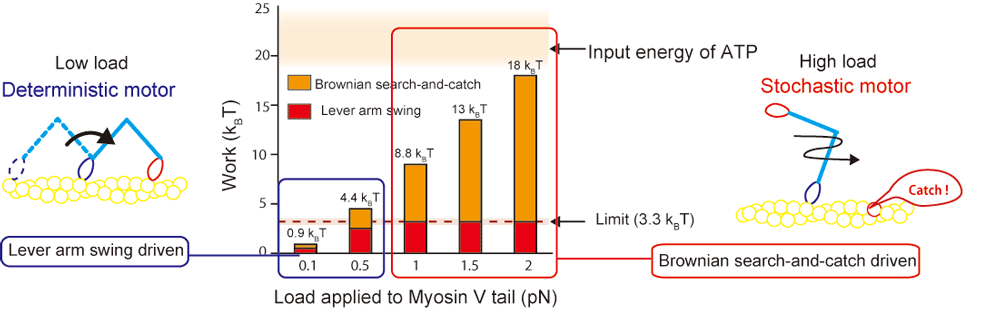
2. Energetics of initiation process for myosin force generation
Detail is linked here. Briefly, we found switching of force generation mode of myosin-V depending on external load. Mainly, two mechanical processes are important for the motor function, one is structural change termed "Lever-arm swing" and the other is rectification of Brownian motion we termed "Brownian search-and-catch". Lever-arm swing is well-known mechanism and written in the popular biology text as a muscle contraction mechanism. Recent single molecule measurements revealed the lever-arm swing but there was less information about energetics (actual measurement data). That is,
- How large work can lever-arm swing actually produce?
- How large work can Brownian search-and-catch produce?
We solved the question by constructing single molecule techniques and DNA molecules as a nano material.
In summary, Lever arm swing contributes to force generation by 15 % of whole work near stall condition. Remaining is driven by the Brownian search-and-catch. We quantified the output work using myosin-V but the distribution of these contribution may be different between myosin molecules (over 30 kinds of myosin exists).
Notable finding here is not only renewing the textbook but stochastic process such as Brownian search-and-catch play a critical role in the function and indicating a very wise nature when we imagine motor function in a cell environment. That is, myosin-V functions as a delivery machine to transport vesicles in a cell. Unlike railway track or turnpike, myosin-V moves along cytoskeletal actin filament where various obstacles such as binding proteins (filamin etc.) exists. In addition, cytosol is very crowded, so, myosin-V must avoid many obstacles in a cell. Because the Brownian search-and-catch is a Brownian process, motor can diffuse between obstacles and search and find appropriate landing position. Therefore, we believe myosin-V can robustly and adaptably transport vesicles in a cell.
Nature's nano machine is designed such that inevitable process by structural change and highly stochastic process by Brownian search-and-catch are hybridized to function efficiently in a cell.
Reference
- “Switching of myosin-V motion between the lever-arm swing and Brownian search-and-catch.”
K. Fujita*, M. Iwaki*†, A. Iwane, L. Marcucci, T. Yanagida
Nature Communications, 3, 956 (2012) (*equally contribution, †corresponding)
Current project
Systems physical biology of Heart・Skeletal muscle (On-going)
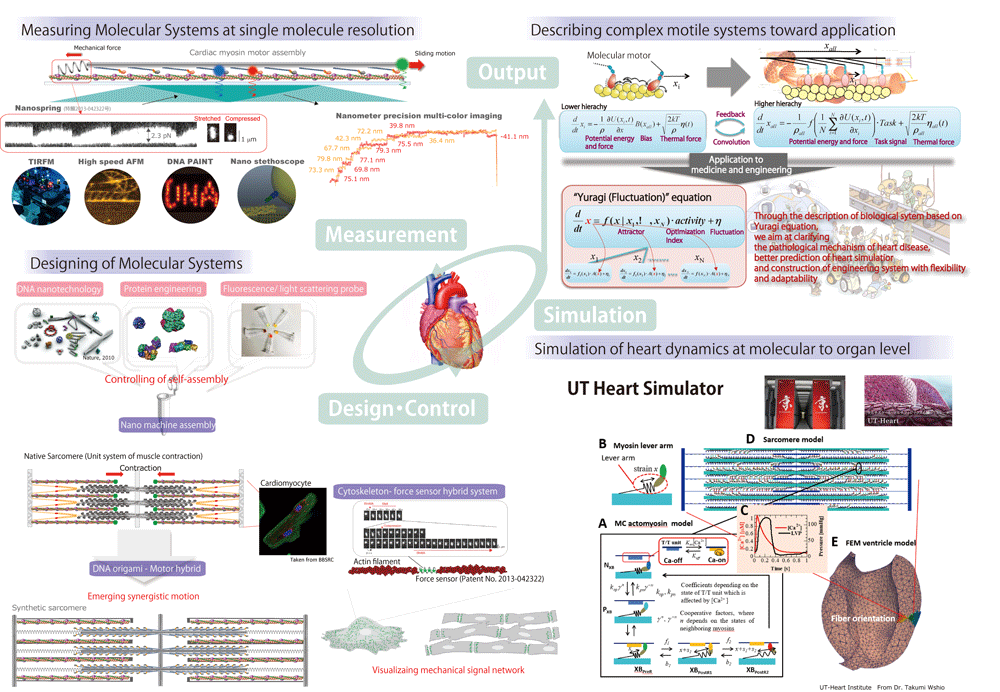
Based on our finding at molecular level, we are challenging to understand the whole heart and skeletal muscle by experimentally connecting molecular dynamics with sarcomere, cardiomyocyte dynamics and by collaboration with precise heart simulator (UT-heart). The systems physical biology is important not only for basic science but also for precise medicine of heart disease such as hypertrophic cardiomyopathy.
To dissect molecular dynamics in macromolecular assembly such as sarcomere at the single molecule level, we utilize DNA nanotechnology (DNA origami), super resolution imaging (DNA PAINT) and high-speed atomic force microscopy.
Detail strategy is shown as follows.
In skeletal and cardiac muscle, there exists highly organized sub cellular module (sarcomere). In sarcomere, motor protein, myosin II is densely packed. Up to now, dynamics of single myosin molecule isolated from sarcomere was well characterized, however, nobody directly observe single myosin dynamics in the sarcomere system. Myosin in sarcomere should mechanically interact with each other and responds to Ca ion signaling, resulting in a cooperative action. The coordination will lead stable and efficient contraction, beating, adaptation against load and robustness. To visualize individual myosin dynamics in sarcomere, we are constructing molecular platform (programmable myosin filament, sarcomere) using DNA origami as a scaffold of sub cellular module.
This approach has various benefits shown below.
- Highly programmable reconstitution of myosin filament and sarcomere is possible (high controllability of spacing, species of myosin)
- We can construct molecular systems which is compatible with single molecule detection techniques such as TIRF, dark-field microscopy and high-speed AFM (high addressability of fluoresce probes to visualize single molecule dynamics etc.)
- Our coarse-grained sarcomere is useful to understand substantial architecture of muscle contraction by adding sarcomere proteins and by increasing the system complexity step-by-step
Sarcomere is mesoscale layer between molecule and cell and clarification of molecular dynamics in sarcomere bridges between single molecule biology and myocyte biology. We combine our experimental results with Heart simulator (UT-Heart developed by the University of Tokyo), which can bridge molecule to organ dynamics in a seamless manner.
Molecular sensor・Super-resolution imaging using DNA nanotechnology (On-going)
We utilize DNA nanotechnology (DNA origami) for our single molecule biophysical studies. DNA origami is a nanotechnology to construct arbitrary nanostructures from DNA and has a huge potential to solve various problems in biology, physics, material science and computer science. The ability of design and scalability of size is rapidly growing, therefore, DNA origami can realize attractive nanoscale devices such as molecular sensor, calculator and nanoactuators etc.
We apply DNA origami not only to muscle research but also to other projects below.
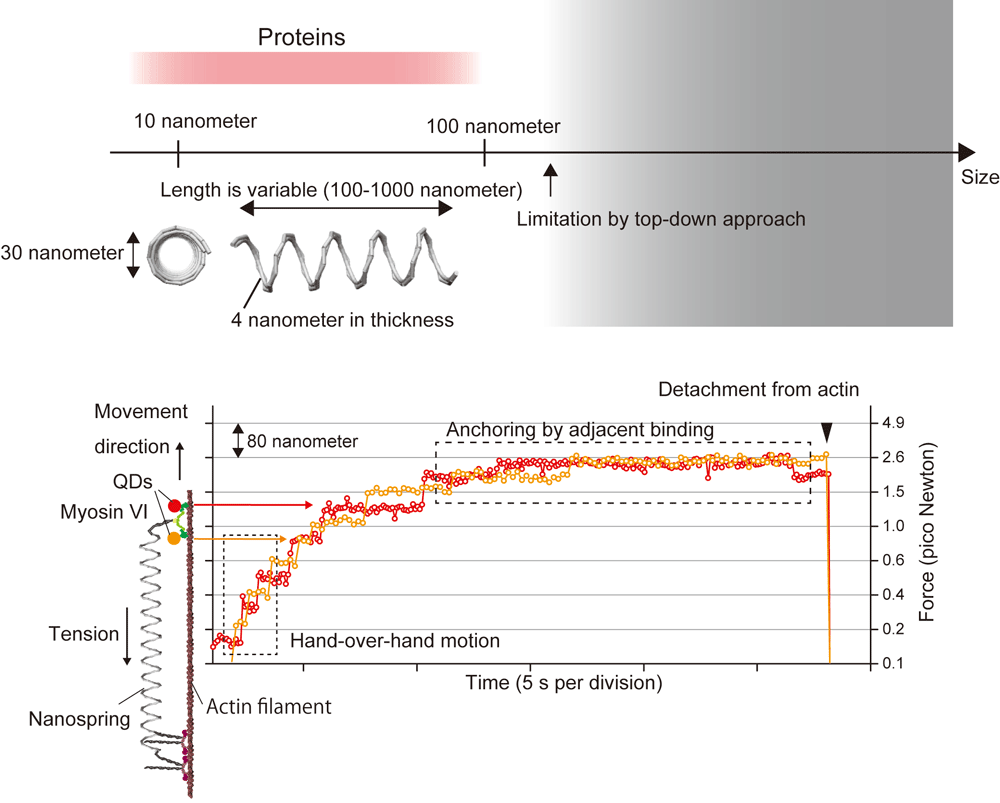
1. Sensing of mechanical force of molecule・cell・tissue by a programmable DNA origami nanospring
We designed coil-shaped nanosized spring using DNA origami technique. The size is similar to protein molecules and advantages are
- Stiffness of spring is tunable (large dynamic range of force sensing)
- High connectivity with biomolecules
- High force resolution due to approximately linear force-extension relation
- High biocompatibility
We have already confirmed usability of nanospring in in vitro experiment (Iwaki et al., Nat. Commun., 2016) and succeeded in observing mechanical interaction between motor proteins and simultaneous observation of force and single molecule dynamics by FIONA or SHREC. Currently, we are trying to attach nanospring to cardiomyocyte and monitor mechanical force (= mechanical signal). In near future, we expect optical control of mechanical force by combining nanospring with photochromic molecules.
Reference
- “A programmable DNA origami nanospring that reveals force-induced adjacent binding of myosin VI heads"
M. Iwaki†, S.F. Wickham, K. Ikezaki, T. Yanagida, W.M. Shih
Nature Communications, 7, 13715 (2016) (†corresponding)
2. Super resolution imaging of macromolecular complex in cell by DNA-PAINT
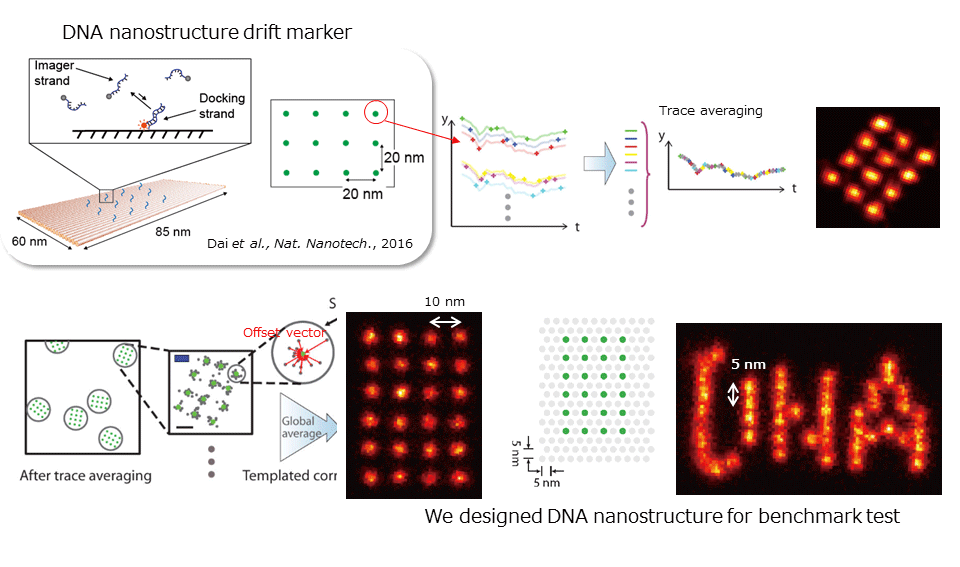
To visualize a non-averaged image of subcellular structure (macromolecular complex), we are developing super resolution imaging technique using DNA origami. We can currently achieve 3-4 nm spatial resolution by DNA-PAINT (DNA-based Point Accumulation for Imaging in Nanoscale Topography) (ref. Dai. et al., Nature Nanotechnology, 2016).
Briefly talking about the technique, this is a kind of single molecule localization microscopy like PALM/STORM, and single molecule photoswitching, image stack and reconstruction is necessary. The photoswitching is normally achieved by laser irradiation, chemical reagent (thiol), spontaneously blinking dye (HMSiR), photoswitchable fluorescent protein (Kohinoor), or short peptide (dye-labeled Lifeact etc.). However, in case of DNA-PAINT, photoswitching is achieved by hybridization kinetics between docking strand, a single stranded oligonucleotide and imager strand, a fluorescent-dye labeled complementary oligonucleotide. When imager strand binds to the docking strand, fluorescent spot appears and when imager strand is dissociated, the spot disappears. By designing the sequence of these oligonucleotides, it achieves high controllability of photoswitching kinetics and easily applicable to variable density clusters. Further, in conventional methods, fluorescent probes photobleach at a certain time and positional determination becomes impossible, but in case of PAINT, there are many imager strands in solution, so repetitive positional determination is possible.
We are currently applying DNA-PAINT to visualize clustering of EGF receptor on membrane and sarcomere, cytoskeletal filament structure in cardiomyocyte.
Nanobio robotics
Under construction





